
Flavin-Photocatalyzed Benzylic Functionalization, Spirocyclization, and Spiroepoxidation of Phenols @pubs.acs.org pubs.acs.org/doi/10.1021/...

Photoexcited Manganese Complex of Abnormal N-Heterocyclic Carbene in Molecular Hydrogen Activation via Hydrogen Atom Transfer Pathway pubs.acs.org/doi/10.1021/...

Photoexcited Manganese Complex of Abnormal N-Heterocyclic Carbene in Molecular Hydrogen Activation via Hydrogen Atom Transfer Pathway pubs.acs.org/doi/10.1021/...
Check out our new paper on a radical strategy to synthesize bicyclo[1.1.1]pentyl C-glycosides !!
Thank you to all the authors and collaborators for this work !
urlr.me/Pnwbj2

Check out our new paper on a radical strategy to synthesize bicyclo[1.1.1]pentyl C-glycosides !!
Thank you to all the authors and collaborators for this work !
urlr.me/Pnwbj2
Florian Doettinger, Jonathan Sagaya, Giacomo Morselli in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...

Florian Doettinger, Jonathan Sagaya, Giacomo Morselli in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
#ChemSky
pubs.acs.org/doi/10.1021/...

#ChemSky
pubs.acs.org/doi/10.1021/...
#ChemSky #sustainability @jacs.acspublications.org
Water as an Electron Donor for Cross-Electrophile Coupling Reactions
pubs.acs.org/doi/10.1021/...

#ChemSky #sustainability @jacs.acspublications.org
Water as an Electron Donor for Cross-Electrophile Coupling Reactions
pubs.acs.org/doi/10.1021/...
pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...
#ChemSky #photocatalysis #metal-free
pubs.acs.org/doi/10.1021/...

#ChemSky #photocatalysis #metal-free
pubs.acs.org/doi/10.1021/...
go.bsky.app/GwH8tnF
(completing the already full OrgChem:
go.bsky.app/NSEFPFJ)
go.bsky.app/GwH8tnF
(completing the already full OrgChem:
go.bsky.app/NSEFPFJ)
MOFs finally got a Nobel Prize!
Read more in this story by my amazing colleague Prachi Patel:
cen.acs.org/people/nobel...

MOFs finally got a Nobel Prize!
Read more in this story by my amazing colleague Prachi Patel:
cen.acs.org/people/nobel...
@indrajitghosh85.bsky.social
doi.org/10.1002/adsc...

@indrajitghosh85.bsky.social
doi.org/10.1002/adsc...
Follow us to stay updated on our latest projects, publications, team activities, and collaboration opportunities.
@ghoshresearchgroup.bsky.social
Follow us to stay updated on our latest projects, publications, team activities, and collaboration opportunities.
@ghoshresearchgroup.bsky.social
#photocatalysis #nanoparticles #ChemSky
doi.org/10.1002/anie...
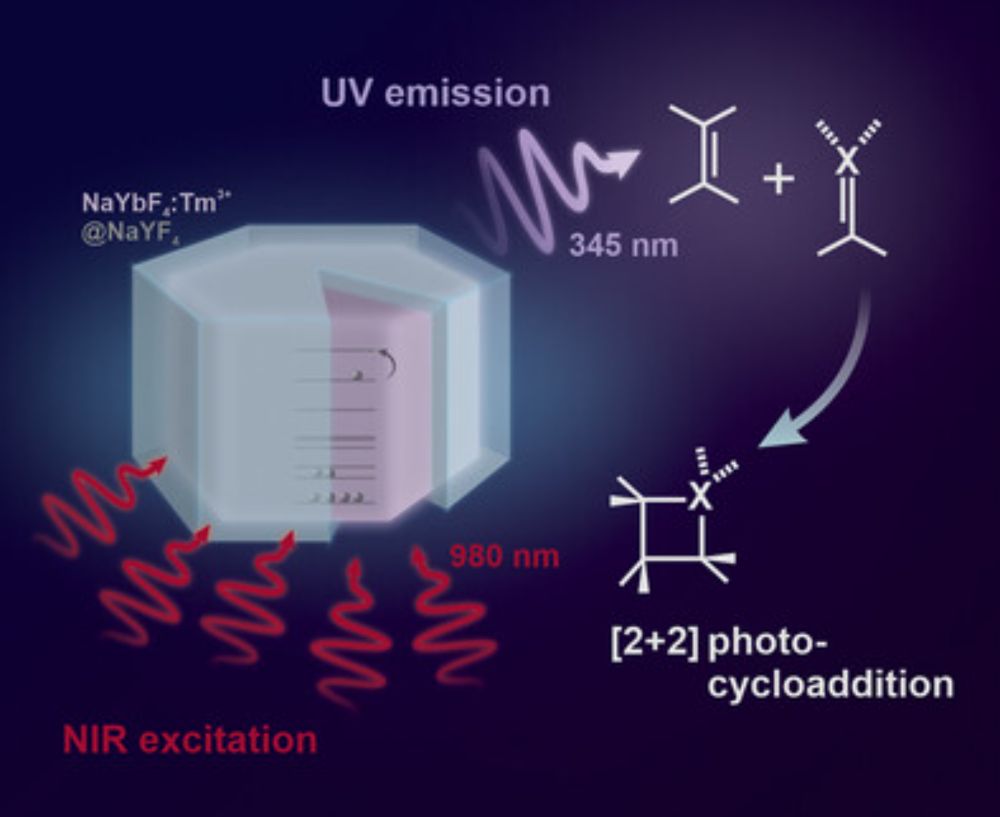
#photocatalysis #nanoparticles #ChemSky
doi.org/10.1002/anie...
Explore the collection here🔽
pubs.rsc.org/en/journals/...

Explore the collection here🔽
pubs.rsc.org/en/journals/...
This review describes the synthesis, structure–property relationships, and examples of applications for seven important classes of photoswitches
doi.org/10.3762/bjoc...

This review describes the synthesis, structure–property relationships, and examples of applications for seven important classes of photoswitches
doi.org/10.3762/bjoc...
chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/...

chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/...
Then use our new strategy to simplify your reactions!
Thanks to all involved!
@angewandtechemie.bsky.social
@indrajitghosh85.bsky.social
#ChemSky #photocatalysis #Nickel
doi.org/10.1002/anie...

Then use our new strategy to simplify your reactions!
Thanks to all involved!
@angewandtechemie.bsky.social
@indrajitghosh85.bsky.social
#ChemSky #photocatalysis #Nickel
doi.org/10.1002/anie...
pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...

Huang et al.: 329 reactions → stirring made little difference.
Cherepanova et al.: stirrers can cause irreproducibility by vial position.
Time to rethink the humble stir bar.
doi.org/10.1021/jacs... #ChemSky

Huang et al.: 329 reactions → stirring made little difference.
Cherepanova et al.: stirrers can cause irreproducibility by vial position.
Time to rethink the humble stir bar.
doi.org/10.1021/jacs... #ChemSky


We’re happy to announce that our research group website is now live! Visit us to stay updated on our latest research projects, publications, team activities, and collaboration opportunities.
sites.google.com/view/ghosh-r...

We’re happy to announce that our research group website is now live! Visit us to stay updated on our latest research projects, publications, team activities, and collaboration opportunities.
sites.google.com/view/ghosh-r...

