
Assistant Professor at ICBAS-University of Porto.
It was a good day! 👏🎉



It was a good day! 👏🎉








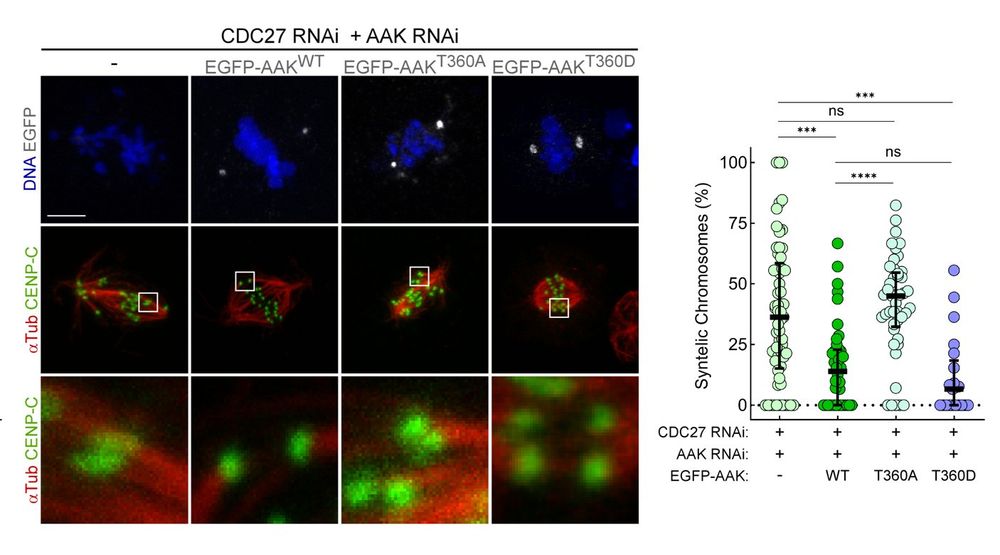
- reduced phosphorylation of the NDC80 tail (NDC80-S67Ph)
- failure to correct faulty kinetochore attachments
- delayed chromosome congression

- reduced phosphorylation of the NDC80 tail (NDC80-S67Ph)
- failure to correct faulty kinetochore attachments
- delayed chromosome congression








