
We could also show that sputum of cystic fibrosis patients contains high levels of MPO functionalised NET-like structures.

We could also show that sputum of cystic fibrosis patients contains high levels of MPO functionalised NET-like structures.
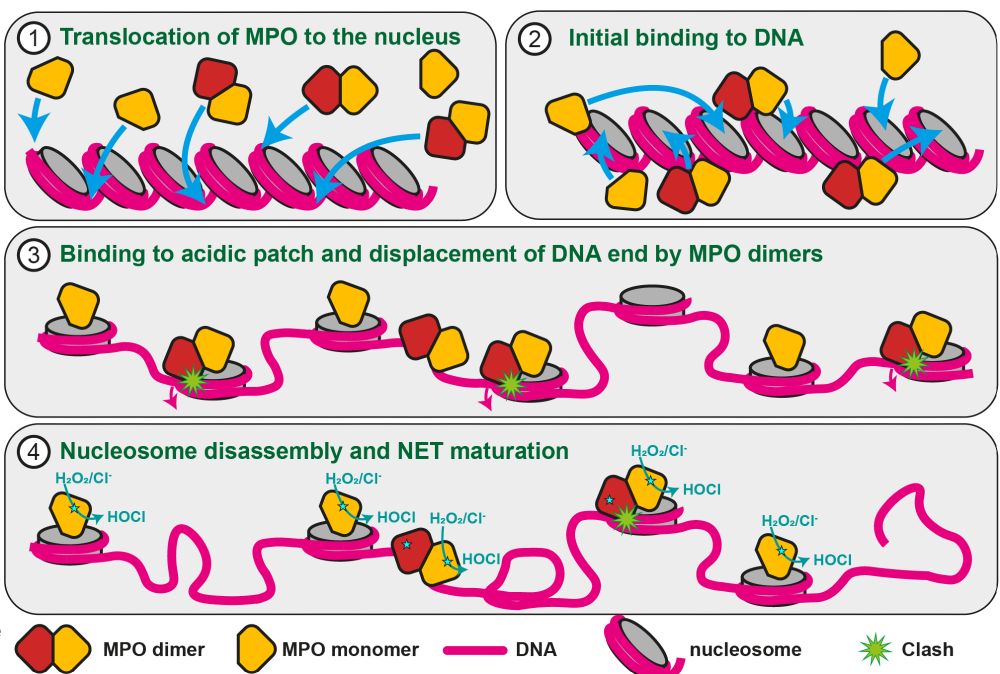
But, unlike dimers, they cannot disassemble the nucleosomes and remain stably associated.
This means that most nucleosomes will be evicted during NETosis, but some are protected by MPO monomers and end up in NETs
But, unlike dimers, they cannot disassemble the nucleosomes and remain stably associated.
This means that most nucleosomes will be evicted during NETosis, but some are protected by MPO monomers and end up in NETs

Indeed, only MPO dimers and not monomers can lead to unwrapping of DNA, making it accessible for nucleases:

Indeed, only MPO dimers and not monomers can lead to unwrapping of DNA, making it accessible for nucleases:
As a consequence, in the presence of MPO dimer the DNA has to locally unwrap and becomes disordered, as visible in this morph:
As a consequence, in the presence of MPO dimer the DNA has to locally unwrap and becomes disordered, as visible in this morph:
But the second MPO unit contacts the DNA at several positions. And this is important for understanding how it can destabilise the nucleosome.

But the second MPO unit contacts the DNA at several positions. And this is important for understanding how it can destabilise the nucleosome.
Not exactly! Instead, it rapidly disassembles nucleosomes when we reconstitute both, leaving behind only MPO bound to DNA! That was a surprise, since we did not add any other factors such as ATP.

Not exactly! Instead, it rapidly disassembles nucleosomes when we reconstitute both, leaving behind only MPO bound to DNA! That was a surprise, since we did not add any other factors such as ATP.
By #CryoEM, we saw that MPO stably binds to the acidic patch of the nucleosome, an a binding mode similar to many other nucleosome interactors.
By #CryoEM, we saw that MPO stably binds to the acidic patch of the nucleosome, an a binding mode similar to many other nucleosome interactors.
This interaction is direct and requires intact nucleosomes.

This interaction is direct and requires intact nucleosomes.
It produces reactive oxygen species and is one of the most important bactericidal enzymes of neutrophils.

It produces reactive oxygen species and is one of the most important bactericidal enzymes of neutrophils.
As #NETs are sticky and functionalised with bactericidal enzymes, they can trap and kill bacteria.

As #NETs are sticky and functionalised with bactericidal enzymes, they can trap and kill bacteria.
And they can form so-called Neutrophil Extracellular Traps (NETs).

And they can form so-called Neutrophil Extracellular Traps (NETs).
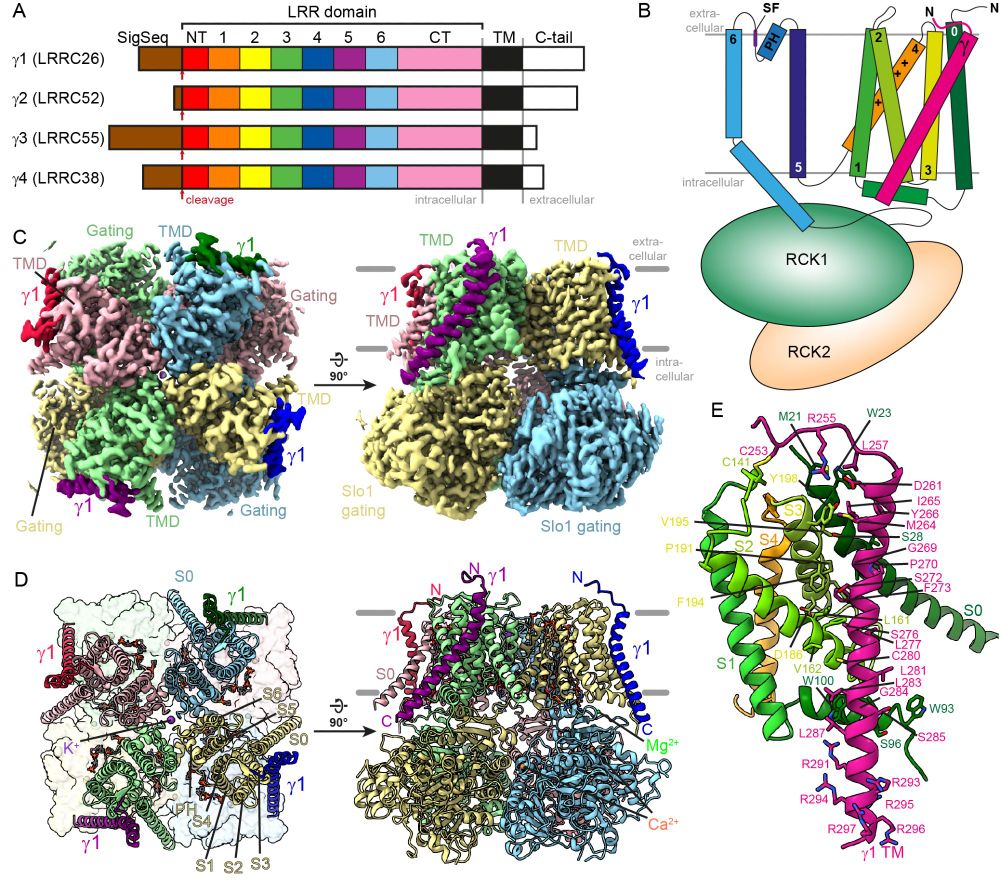
This stretch could locally change the voltage potential across the membrane and allow Slo1 to open without membrane depolarization!

This stretch could locally change the voltage potential across the membrane and allow Slo1 to open without membrane depolarization!
biorxiv.org/content/10.1...
We determined the CryoEM structure of the Slo1 potassium channel in complex with the regulatory subunit γ1 and propose a mechanism of how γ1 can activate Slo1.
biorxiv.org/content/10.1...
We determined the CryoEM structure of the Slo1 potassium channel in complex with the regulatory subunit γ1 and propose a mechanism of how γ1 can activate Slo1.
We focus on structures and mechanisms and try to reveal similarities in modes of action between chemically diverse compounds. In the end, many act surprisingly similar!
rdcu.be/doHyK

We focus on structures and mechanisms and try to reveal similarities in modes of action between chemically diverse compounds. In the end, many act surprisingly similar!
rdcu.be/doHyK

