#maingroup #inorganic #polymers #materials #coordinationchemistry #chemsky
https://chitnislab.ca/
https://orcid.org/0000-0001-9180-7907
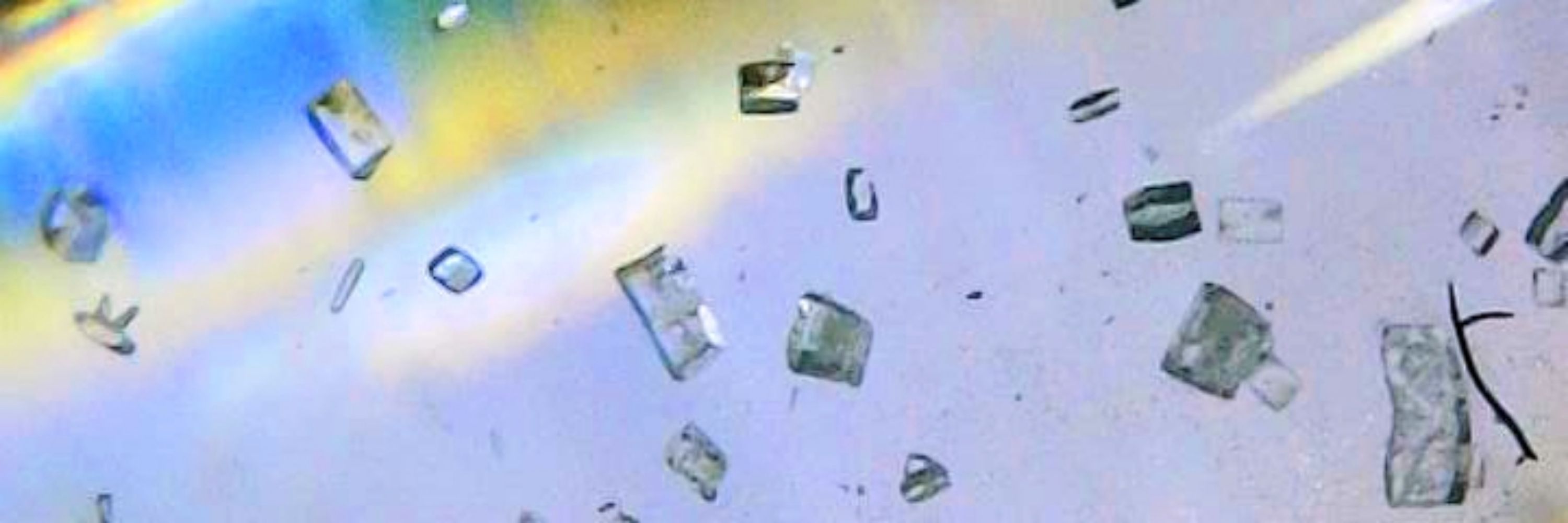
I'm too afraid to take dmso over 100C so will skip that one.
I'm too afraid to take dmso over 100C so will skip that one.
Ideally, we would remove the solvent by washing it away with hydrocarbons/diethyl ether.
Ideally, we would remove the solvent by washing it away with hydrocarbons/diethyl ether.

