kempler.uoregon.edu | electrochemistry.uoregon.edu/
It will take a while to bring down costs, but this is inevitable. It makes too much sense.

It will take a while to bring down costs, but this is inevitable. It makes too much sense.
Great way to kick off the fall quarter with a new cohort of electrochemistry students here at Oregon.

Great way to kick off the fall quarter with a new cohort of electrochemistry students here at Oregon.
This was a fun collaboration with Arun Devaraj's team at PNNL... full paper here: pubs.acs.org/doi/10.1021/...

This was a fun collaboration with Arun Devaraj's team at PNNL... full paper here: pubs.acs.org/doi/10.1021/...
Details can be found here: www.epfl.ch/labs/alchemy...
#chemsky #matsky

Details can be found here: www.epfl.ch/labs/alchemy...
#chemsky #matsky
www.bbc.co.uk/news/article...

www.bbc.co.uk/news/article...

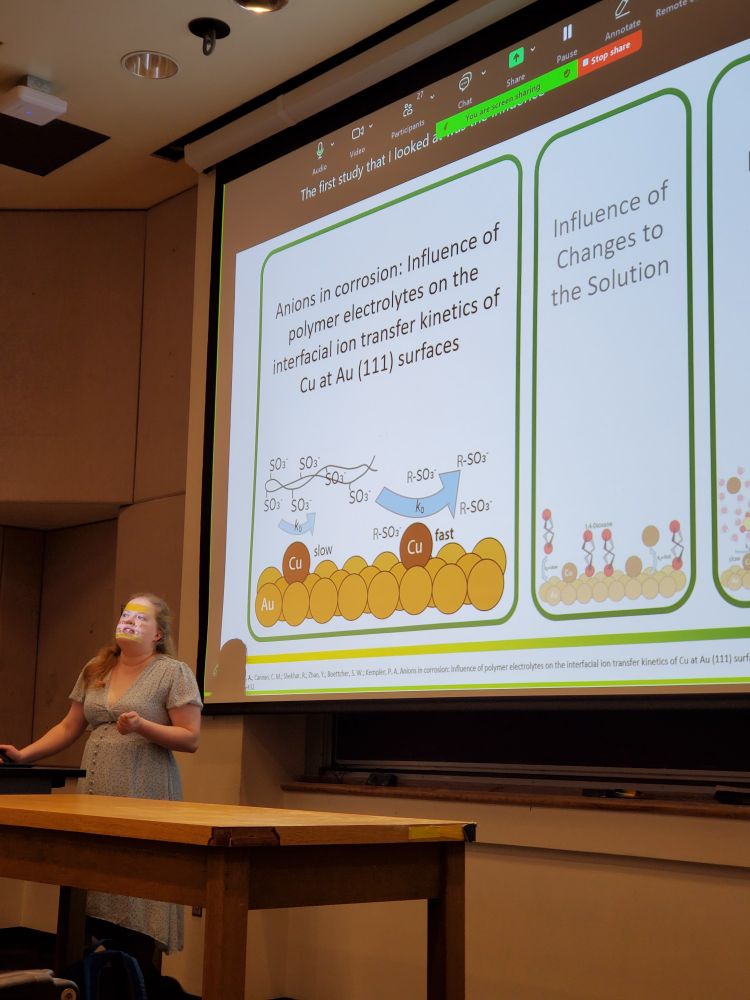
Pathways to Electrochemical Ironmaking at Scale Via the Direct Reduction of Fe2O3 | ACS Energy Letters pubs.acs.org/doi/10.1021/...
Pathways to Electrochemical Ironmaking at Scale Via the Direct Reduction of Fe2O3 | ACS Energy Letters pubs.acs.org/doi/10.1021/...
mse.nd.edu/research/sma...

mse.nd.edu/research/sma...

pubs.acs.org/doi/full/10....
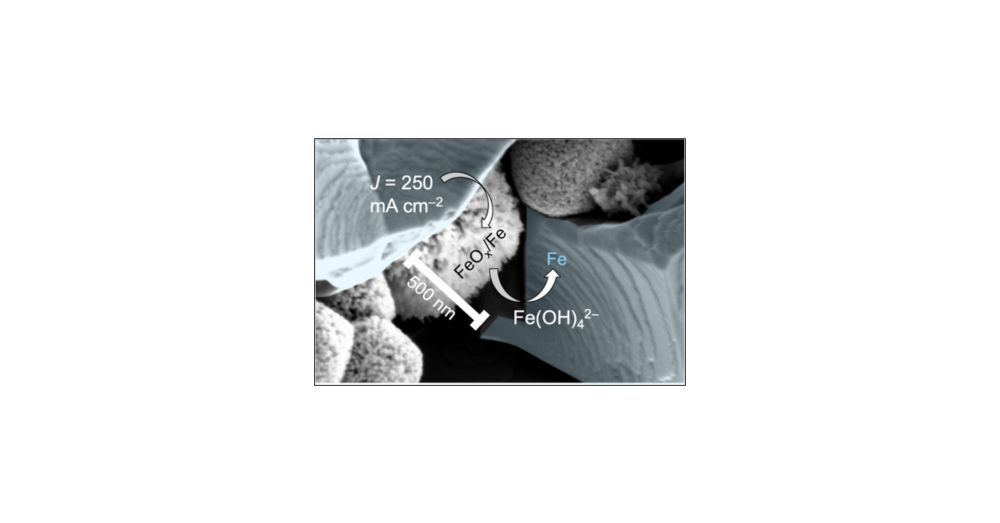
Here, Manasa used methods pioneered by the @boettcherlab.bsky.social to rigorously control dissolved iron in a zero-gap alkaline electrolysis cell.
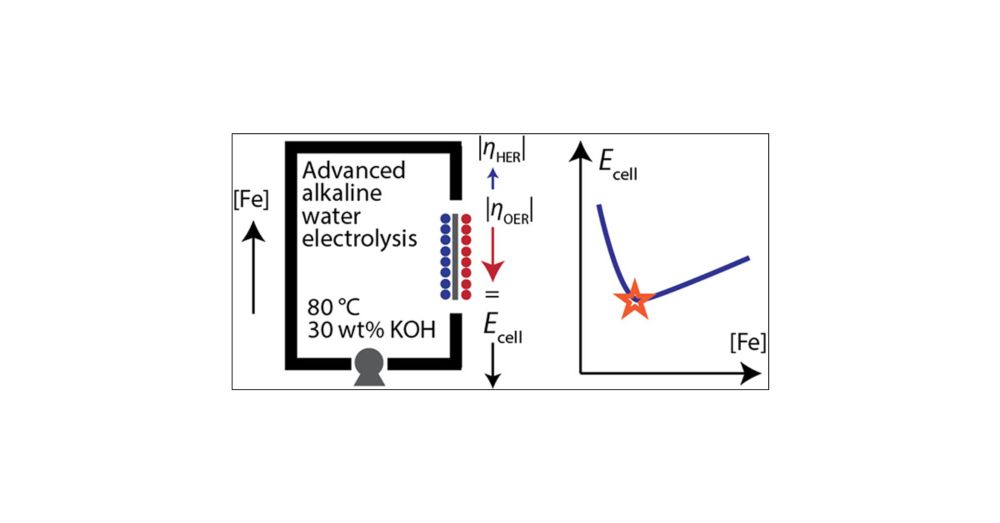
pubs.acs.org/doi/full/10....
Here, Manasa used methods pioneered by the @boettcherlab.bsky.social to rigorously control dissolved iron in a zero-gap alkaline electrolysis cell.
pubs.acs.org/doi/full/10....
Kira showed us that the intrinsic exchange rate of Cu2+ at Au(111) is suppressed by polymer electrolytes. Important implications for catalyst durability!
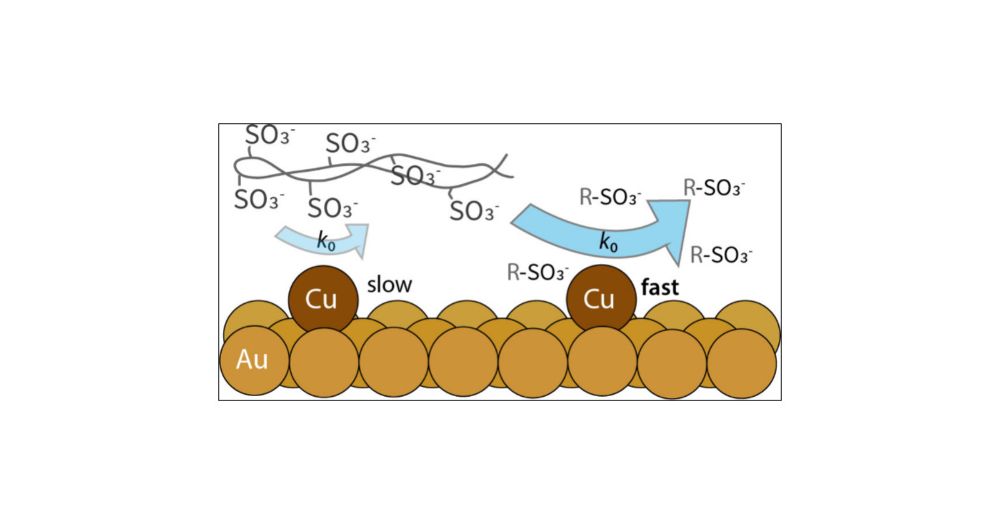
pubs.acs.org/doi/full/10....
Kira showed us that the intrinsic exchange rate of Cu2+ at Au(111) is suppressed by polymer electrolytes. Important implications for catalyst durability!
Let us know if you'd like to see some resumes and please share for visibility 🧪⚡
Let us know if you'd like to see some resumes and please share for visibility 🧪⚡
go.bsky.app/UzqjkNk
go.bsky.app/UzqjkNk

go.bsky.app/2Um4Ubc
go.bsky.app/2Um4Ubc
Currently curious about conversion reactions between metals/metal oxides... particularly for green steel, aqueous batteries, and electrolyzers powered by intermittent power ⚡

Currently curious about conversion reactions between metals/metal oxides... particularly for green steel, aqueous batteries, and electrolyzers powered by intermittent power ⚡

