For now, combination therapy has won the bragging rights, but I would not go so far as to proclaim this is a practice-changing trial.
For now, combination therapy has won the bragging rights, but I would not go so far as to proclaim this is a practice-changing trial.
No clearcut evidence that hyperkalemia risk is mitigated by combining sglt2i with nsMRA (? Inadequate power/Sample Size)
No clearcut evidence that hyperkalemia risk is mitigated by combining sglt2i with nsMRA (? Inadequate power/Sample Size)
Lipid hypothesis lingered on for several decades until LDL-C lowering was proven to reduce CV risk. Analogy applies to INOCA/ANOCA/MINOCA. Need novel targeted Rxs.
Lipid hypothesis lingered on for several decades until LDL-C lowering was proven to reduce CV risk. Analogy applies to INOCA/ANOCA/MINOCA. Need novel targeted Rxs.
Is it still a null outcome?
Is it still a null outcome?
When should it be considered first-line therapy?
Does the proposed cost provide value?
When should it be considered first-line therapy?
Does the proposed cost provide value?
Trial met NI despite the fact that HR excluded 1.0, i.e., MitraClip was INFERIOR to surgery, yet NONINFERIOR!
www.nejm.org/doi/full/10....
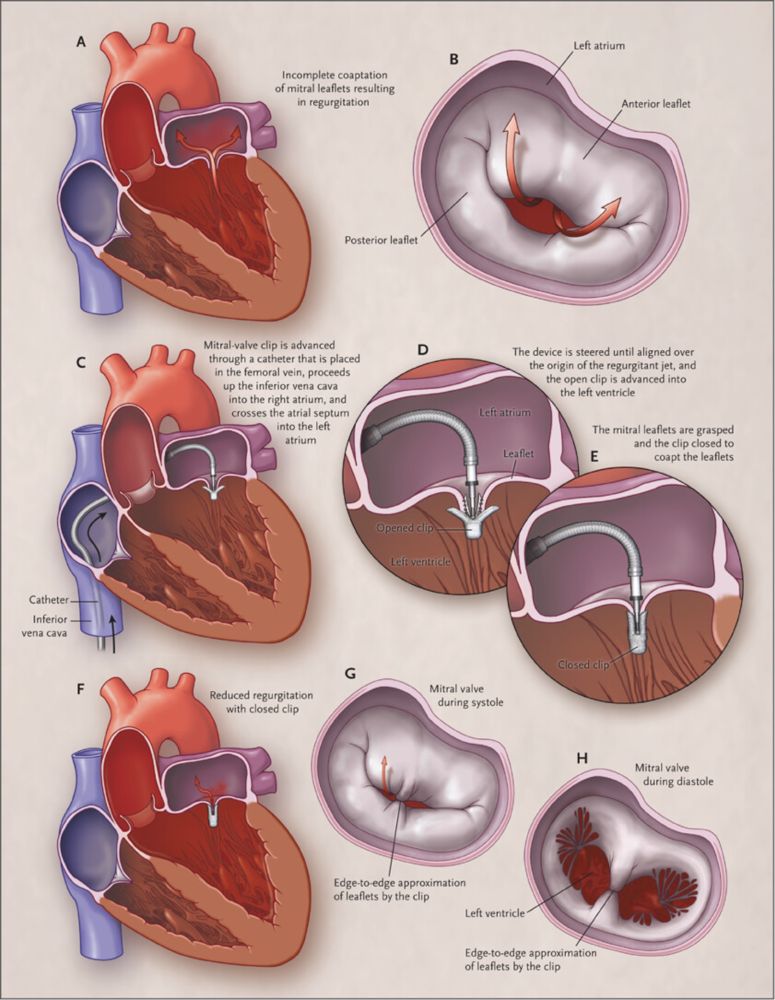
Trial met NI despite the fact that HR excluded 1.0, i.e., MitraClip was INFERIOR to surgery, yet NONINFERIOR!
www.nejm.org/doi/full/10....
Key Qs
What is probability of success with obicetrapib?
Why should obicetrapib succeed when 2 other CETPi did not?
Is absolute or relative LDL reduction key driver of benefit?
Is duration of treatment important?
Is LDL lowering with CETPi different from other LDL lowering Rx?
Key Qs
What is probability of success with obicetrapib?
Why should obicetrapib succeed when 2 other CETPi did not?
Is absolute or relative LDL reduction key driver of benefit?
Is duration of treatment important?
Is LDL lowering with CETPi different from other LDL lowering Rx?
PREVAIL(Obicetrapib), N=9541, F/U: ~4y
Mean LDL 103; LDL⬇️35-40%, Lp(a)⬇️45% (based on BROOKLYN)
PEP (4-MACE): Powered for 15-20% RRR
Recruitment completed; event-driven trial
Targeting higher baseline LDL & higher risk patients
PREVAIL(Obicetrapib), N=9541, F/U: ~4y
Mean LDL 103; LDL⬇️35-40%, Lp(a)⬇️45% (based on BROOKLYN)
PEP (4-MACE): Powered for 15-20% RRR
Recruitment completed; event-driven trial
Targeting higher baseline LDL & higher risk patients
REVEAL (Anacetrapib), N=30,449, F/U: 4.1y
Mean LDL 61; LDL⬇️41%, Lp(a)⬇️25%
PEP (3-MACE): 10.8% vs 11.8%, HR 0.91, 0.85-0.97
Development halted (long half-life, modest Rx effect)
REVEAL (Anacetrapib), N=30,449, F/U: 4.1y
Mean LDL 61; LDL⬇️41%, Lp(a)⬇️25%
PEP (3-MACE): 10.8% vs 11.8%, HR 0.91, 0.85-0.97
Development halted (long half-life, modest Rx effect)

