






















www.federalregister.gov/agencies/nat...

www.federalregister.gov/agencies/nat...
pmc.ncbi.nlm.nih.gov/articles/PMC...
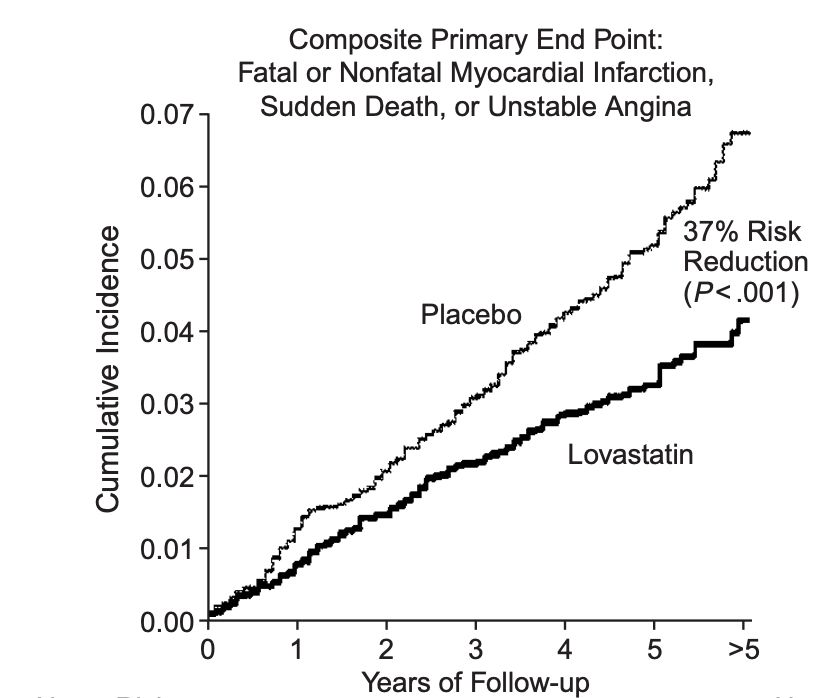
pmc.ncbi.nlm.nih.gov/articles/PMC...
pubmed.ncbi.nlm.nih.gov/6262757/
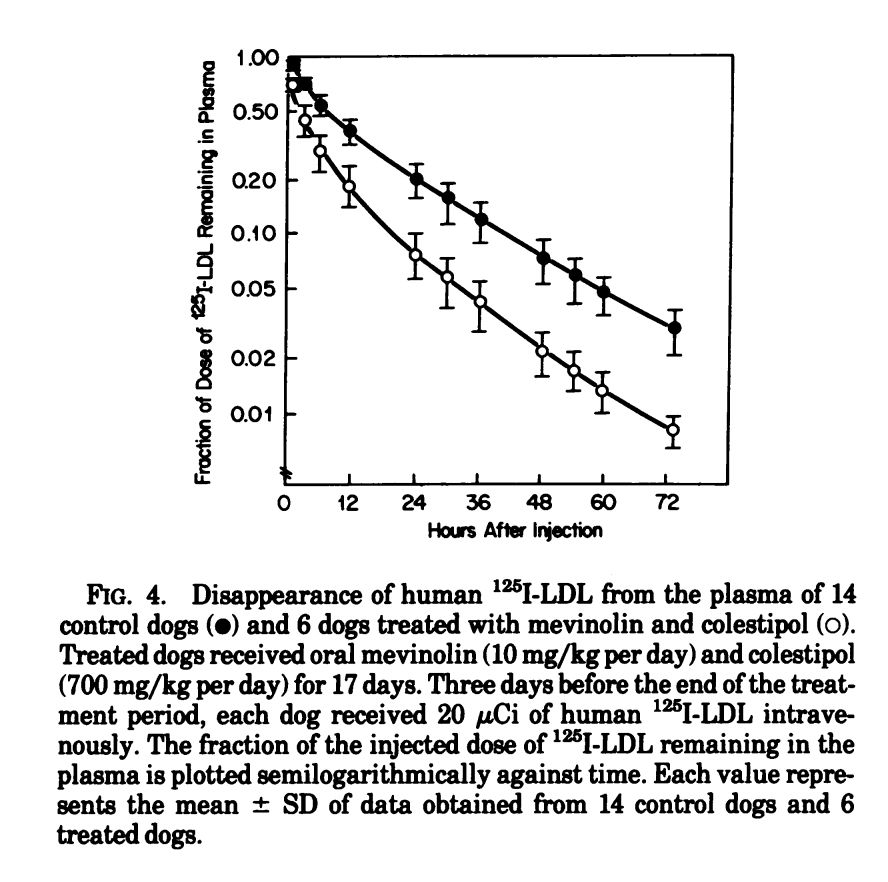
pubmed.ncbi.nlm.nih.gov/6262757/
www.sciencedirect.com/science/arti...
www.sciencedirect.com/science/arti...

