
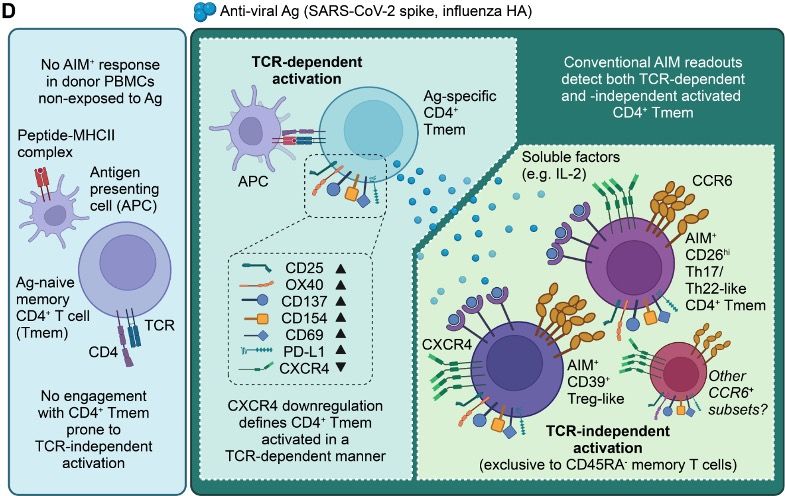
He was able to show that ag-specific CD4 cells downregulate CXCR4 while upregulating 4-1BB/CD137, giving us a more accurate way to define antigen specificity.
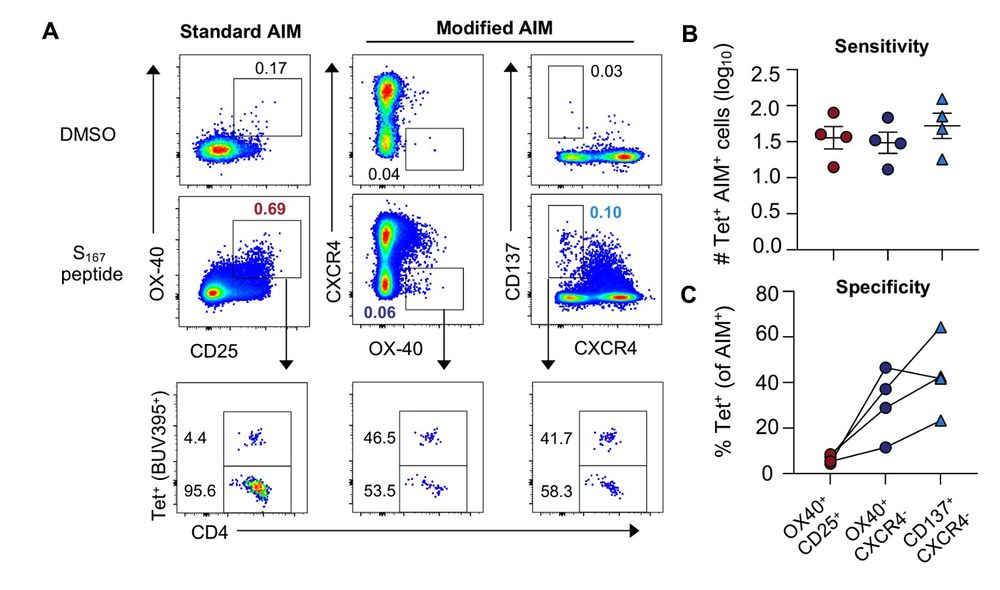
He was able to show that ag-specific CD4 cells downregulate CXCR4 while upregulating 4-1BB/CD137, giving us a more accurate way to define antigen specificity.
These same subsets are also found during standard peptide-based AIM assays.
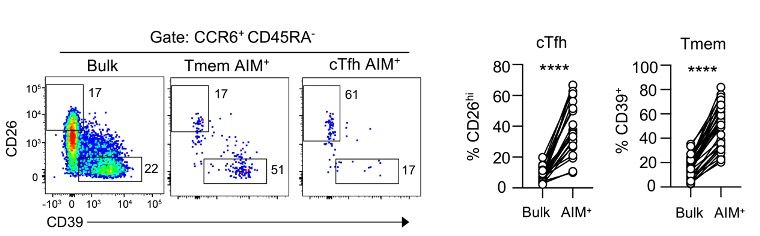
These same subsets are also found during standard peptide-based AIM assays.
In a transwell assay, anti-CD3/CD28 stimulation can drive T cells in an adjacent chamber to express common activation markers (CD25, OX-40, 4-1BB, CD40L).
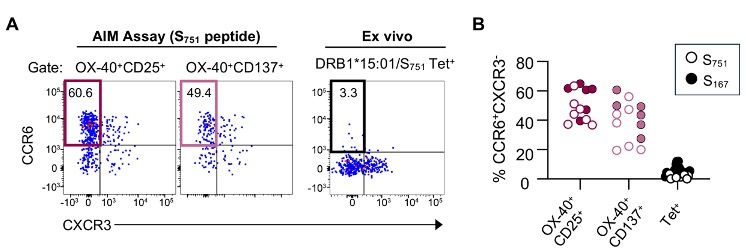
All of these responding cells had a memory phenotype, and were strongly enriched for CCR6 expression.
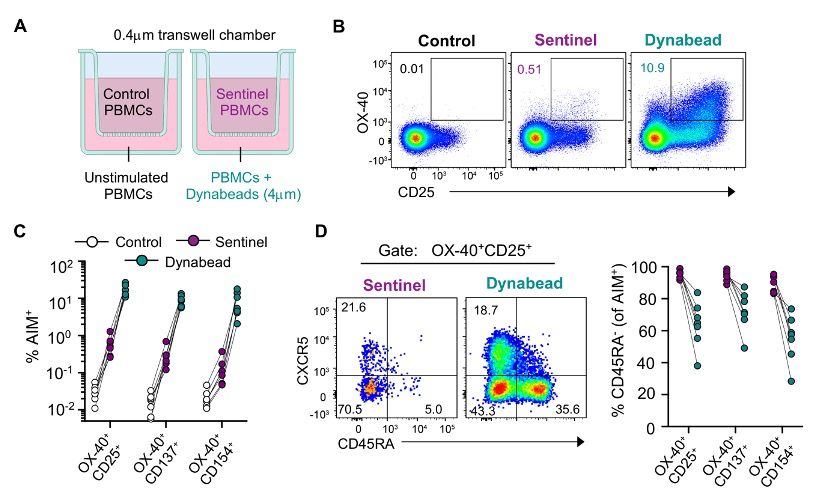
In a transwell assay, anti-CD3/CD28 stimulation can drive T cells in an adjacent chamber to express common activation markers (CD25, OX-40, 4-1BB, CD40L).
All of these responding cells had a memory phenotype, and were strongly enriched for CCR6 expression.
Why?
Why?

