
mugridgelab.org




Looking forward to this GRC!

Looking forward to this GRC!
We're working on more pieces of this puzzle to show how Elp3/Elongator tRNA modification is regulated!
Congrats Evan! 9/9

We're working on more pieces of this puzzle to show how Elp3/Elongator tRNA modification is regulated!
Congrats Evan! 9/9










Photos… 🧵


Photos… 🧵

💡TRMT1 cleavage & inactivation may be another mechanism by which SARS-CoV-2 manipulates host tRNA modifications and translation!
🦠We think this could impact viral pathogenesis or phenotypes!
💊Our structure might help understand Mpro substrate recognition or different pockets to drug!
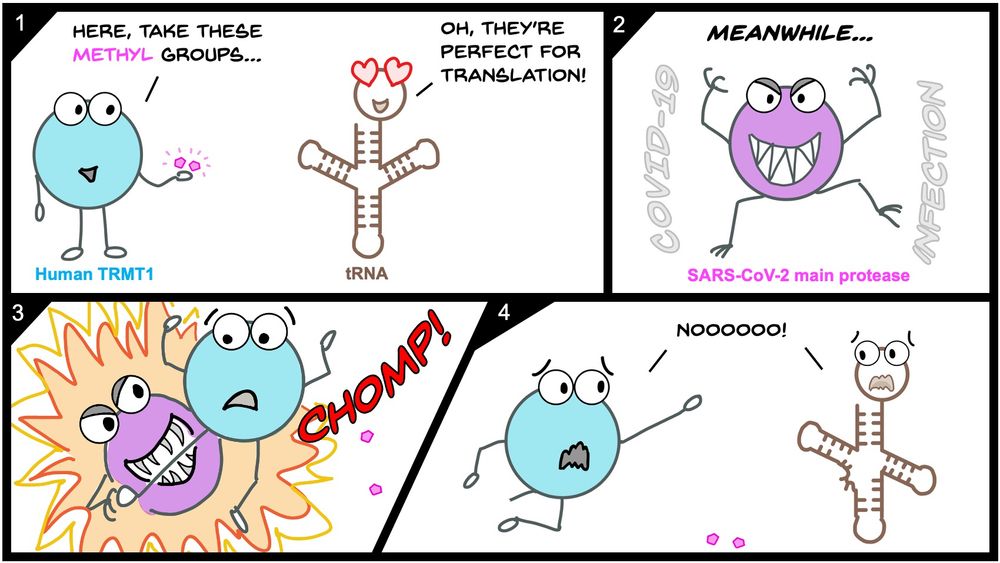
💡TRMT1 cleavage & inactivation may be another mechanism by which SARS-CoV-2 manipulates host tRNA modifications and translation!
🦠We think this could impact viral pathogenesis or phenotypes!
💊Our structure might help understand Mpro substrate recognition or different pockets to drug!
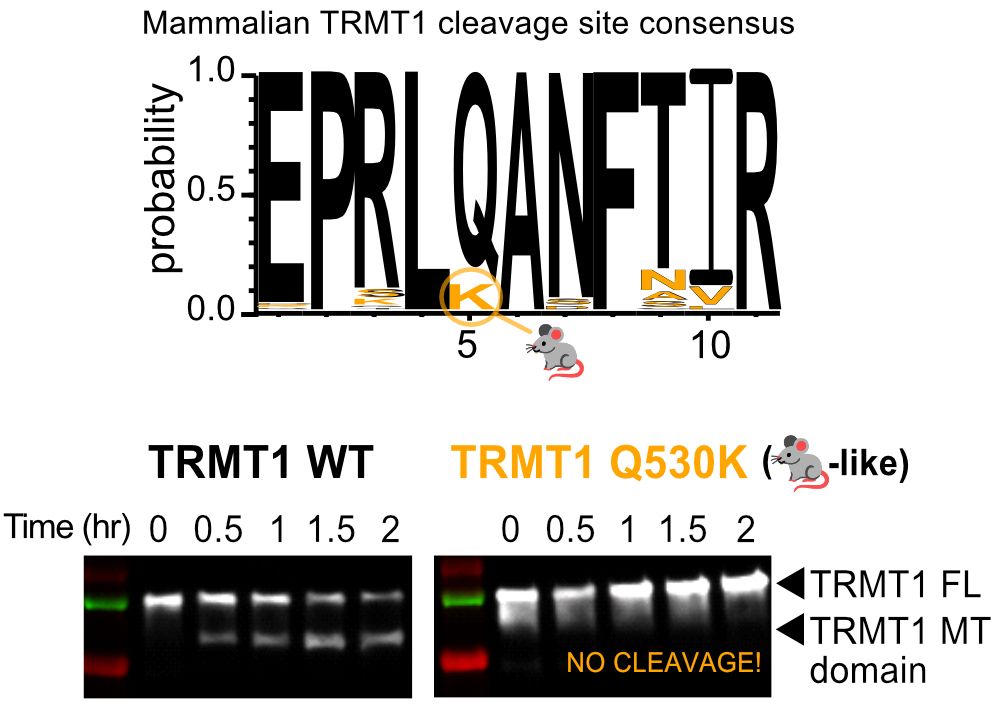

The tRNA mods installed by TRMT1 are important for translation and redox homeostasis, so this could have impacts during infection!... 4/9

The tRNA mods installed by TRMT1 are important for translation and redox homeostasis, so this could have impacts during infection!... 4/9

















