
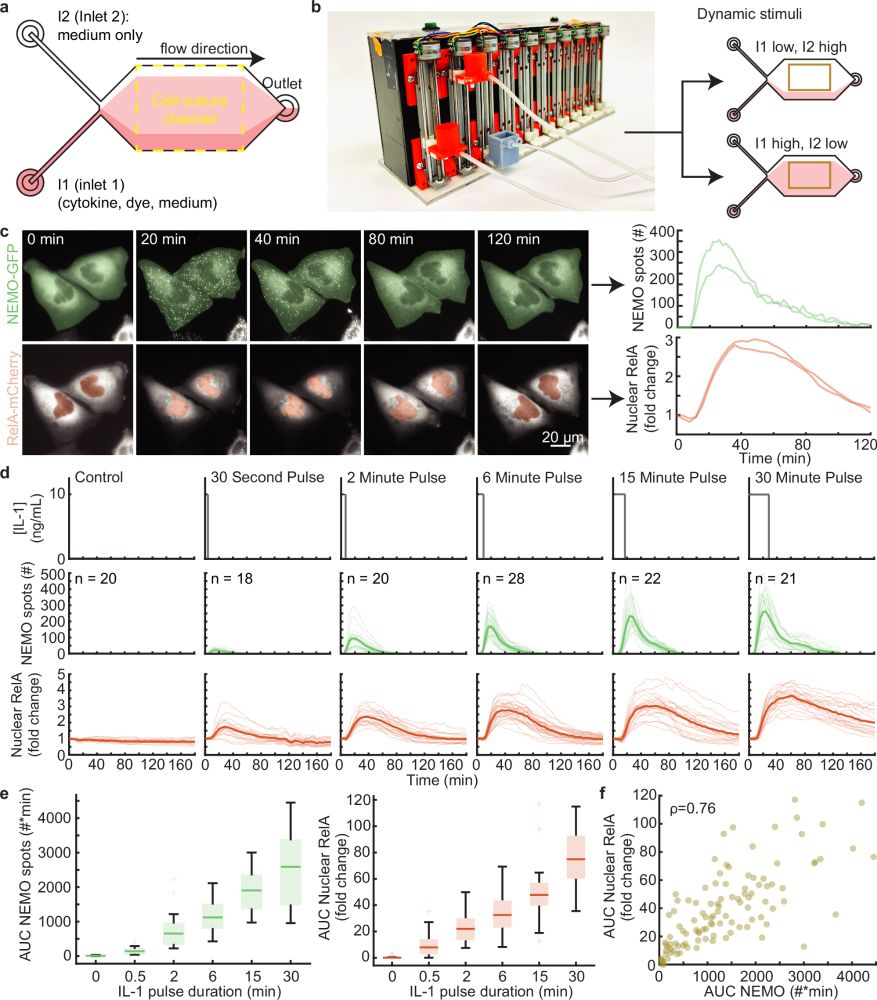
A new study from Steven Smeal and Robin E.C. Lee reveals the timing of molecular signals can change how cells respond to their environment, with implications for cancer treatment and drug discovery.
Read more: rdcu.be/ezTxF

A new study from Steven Smeal and Robin E.C. Lee reveals the timing of molecular signals can change how cells respond to their environment, with implications for cancer treatment and drug discovery.
Read more: rdcu.be/ezTxF
@cmupittcompbio.bsky.social
PhD student Steven Smeal and all the co-authors for their critical contributions.

@cmupittcompbio.bsky.social
PhD student Steven Smeal and all the co-authors for their critical contributions.
www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...

