Isaac Lamb, MD
@isaaclamb01.bsky.social
12 followers
44 following
82 posts
PGY-4 neurology resident. Interested in clinical neurology, medical education, history, and nerd stuff.
Posts
Media
Videos
Starter Packs
Isaac Lamb, MD
@isaaclamb01.bsky.social
· Jul 12
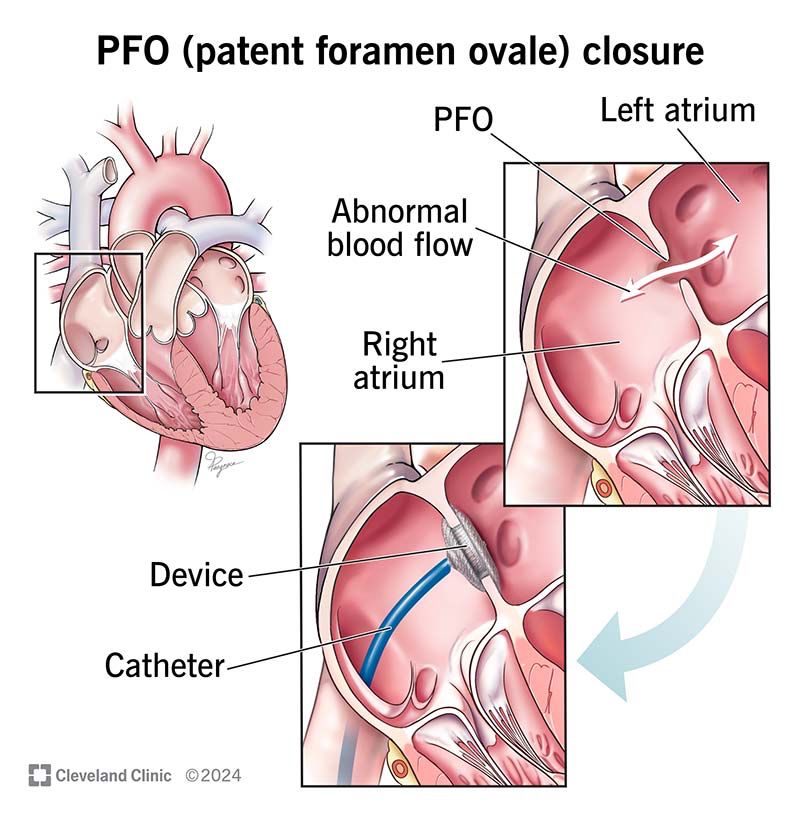
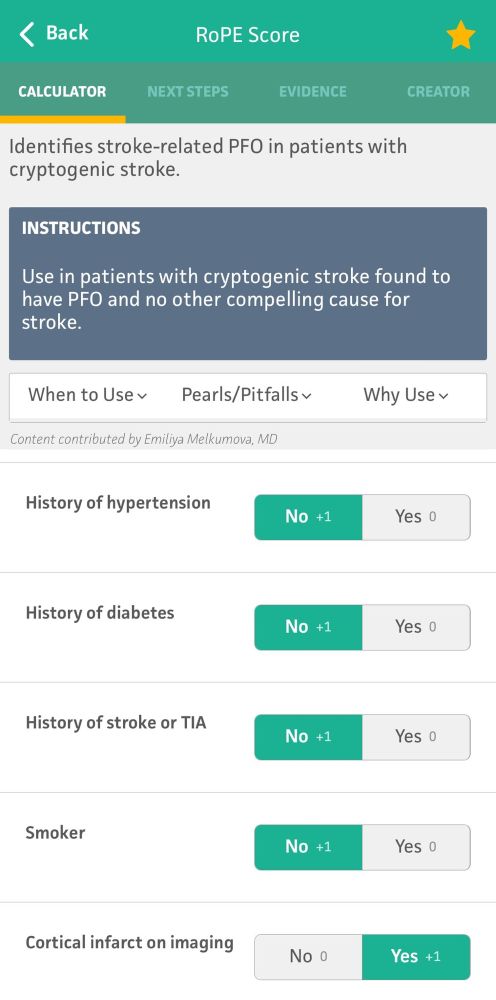
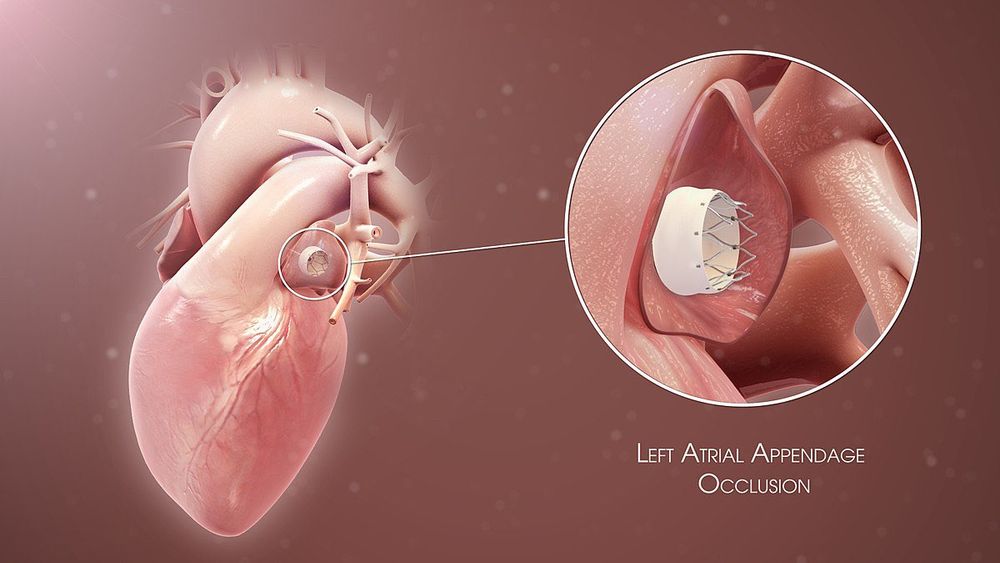
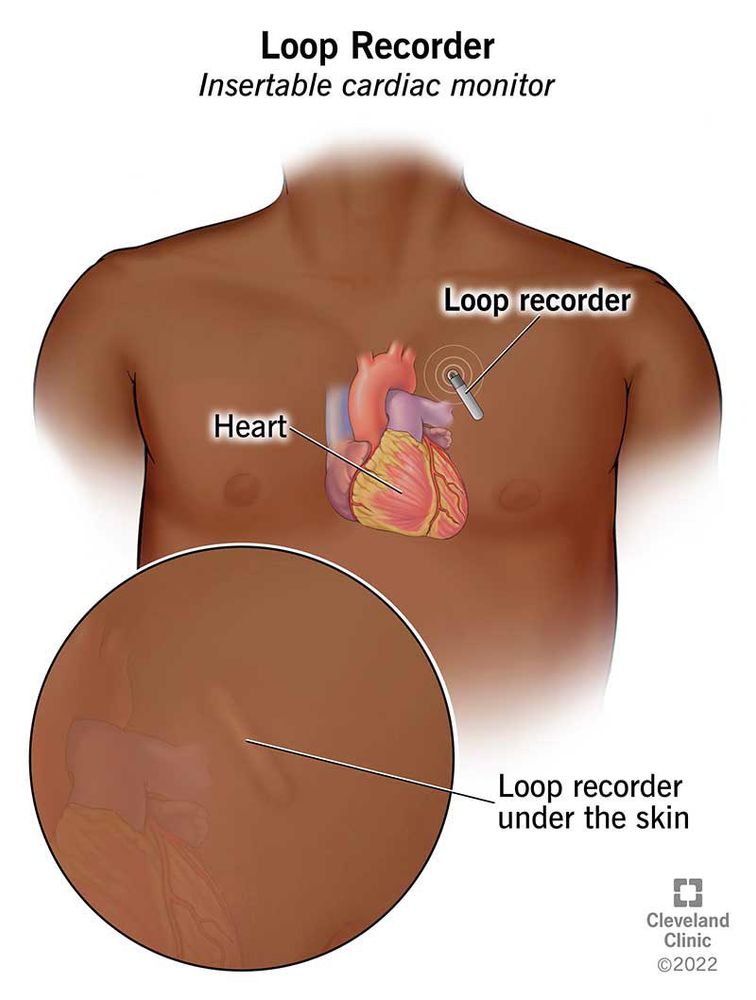
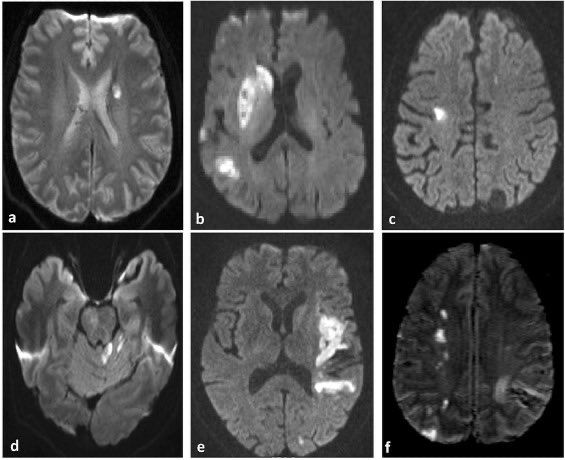
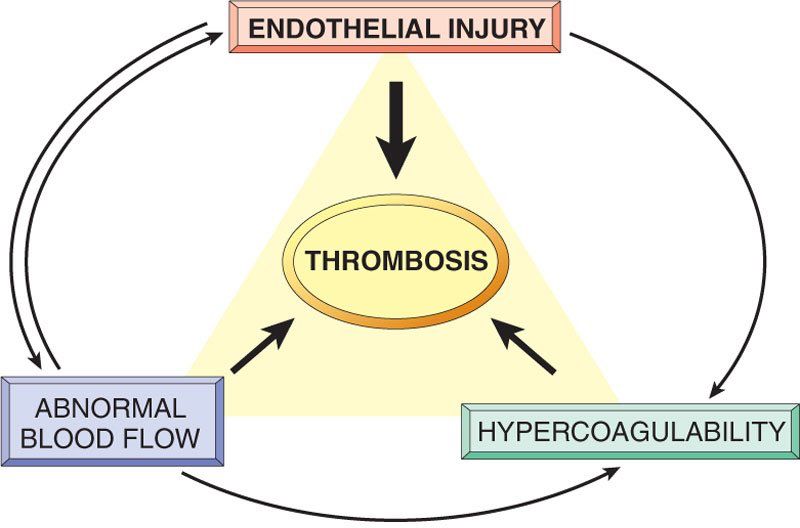
Patent Foramen Ovale: What It Is and How Serious It Can Be
Patent foramen ovale is a normal feature in your newborn’s heart. But when they still have it past age 3, it might need treatment. Learn more about this condition and its treatment.
my.clevelandclinic.org
Isaac Lamb, MD
@isaaclamb01.bsky.social
· Jul 12
Isaac Lamb, MD
@isaaclamb01.bsky.social
· Jul 12
Isaac Lamb, MD
@isaaclamb01.bsky.social
· Jul 12
Isaac Lamb, MD
@isaaclamb01.bsky.social
· Jul 12
Isaac Lamb, MD
@isaaclamb01.bsky.social
· Jul 12
Isaac Lamb, MD
@isaaclamb01.bsky.social
· Jul 12
Isaac Lamb, MD
@isaaclamb01.bsky.social
· Jul 12
Isaac Lamb, MD
@isaaclamb01.bsky.social
· Jul 12
Isaac Lamb, MD
@isaaclamb01.bsky.social
· Jul 12
Isaac Lamb, MD
@isaaclamb01.bsky.social
· Jul 12
Isaac Lamb, MD
@isaaclamb01.bsky.social
· Jul 12
Isaac Lamb, MD
@isaaclamb01.bsky.social
· Jul 12