
👉Read the recap: www.bloodpac.org/bloodpac-blo...
👉Watch the recording: www.bloodpac.org/performance-...

👉Read the recap: www.bloodpac.org/bloodpac-blo...
👉Watch the recording: www.bloodpac.org/performance-...
Our Executive Director, Lauren Leiman, moderated “Demystifying MRD with BLOODPAC,” joined by leaders from Illumina, Harvard, BMS & BLOODPAC to discuss best practices and innovation in cancer monitoring. #MRD #LiquidBiopsy #Oncology

Our Executive Director, Lauren Leiman, moderated “Demystifying MRD with BLOODPAC,” joined by leaders from Illumina, Harvard, BMS & BLOODPAC to discuss best practices and innovation in cancer monitoring. #MRD #LiquidBiopsy #Oncology
🔗 nrichdx.com

🔗 nrichdx.com
Join experts from Illumina, HMS, BMS & BLOODPAC to explore the future of MRD testing.
#LiquidBiopsy #Oncology

Join experts from Illumina, HMS, BMS & BLOODPAC to explore the future of MRD testing.
#LiquidBiopsy #Oncology


The BLOODPAC New Frontiers Seminar happens this week, November 5, 1–3 PM ET.
Join us to explore how multimodal data is transforming therapy selection and advancing personalized cancer care.
🎟️ Register here: bit.ly/BP-New-Front...
The BLOODPAC New Frontiers Seminar happens this week, November 5, 1–3 PM ET.
Join us to explore how multimodal data is transforming therapy selection and advancing personalized cancer care.
🎟️ Register here: bit.ly/BP-New-Front...
🗓️ November 5 | 1–3 PM ET
🎟️ Register here: bit.ly/BP-New-Front...


🗓️ November 5 | 1–3 PM ET
🎟️ Register here: bit.ly/BP-New-Front...
👉 2025 seminar registration: bit.ly/BP-New-Front...
👉 Watch the 2024 seminar: www.bloodpac.org/beyond-ctdna

👉 2025 seminar registration: bit.ly/BP-New-Front...
👉 Watch the 2024 seminar: www.bloodpac.org/beyond-ctdna
The BLOODPAC New Frontiers Seminar returns on November 5. This year, we’re tackling one of the most important frontiers in precision oncology: using multimodal data for therapy selection.
Save your spot today ➡️ bit.ly/BP-New-Front...

The BLOODPAC New Frontiers Seminar returns on November 5. This year, we’re tackling one of the most important frontiers in precision oncology: using multimodal data for therapy selection.
Save your spot today ➡️ bit.ly/BP-New-Front...

👉 2025 seminar registration: bit.ly/BP-New-Front...
👉 Watch the 2024 seminar: www.bloodpac.org/beyond-ctdna

👉 2025 seminar registration: bit.ly/BP-New-Front...
👉 Watch the 2024 seminar: www.bloodpac.org/beyond-ctdna
👉Blog: bit.ly/4q4lnR4
👉Full Paper: bit.ly/MRDlexicon

👉Blog: bit.ly/4q4lnR4
👉Full Paper: bit.ly/MRDlexicon
👉 Register for the 2025 seminar: bit.ly/BP-New-Front...
👉 Last year seminar: www.bloodpac.org/beyond-ctdna

👉 Register for the 2025 seminar: bit.ly/BP-New-Front...
👉 Last year seminar: www.bloodpac.org/beyond-ctdna
📆 𝙉𝙤𝙫𝙚𝙢𝙗𝙚𝙧 5 | 1–3 𝙋𝙈 𝙀𝙏
🎯 “𝙉𝙚𝙬 𝙁𝙧𝙤𝙣𝙩𝙞𝙚𝙧𝙨 𝙞𝙣 𝙏𝙝𝙚𝙧𝙖𝙥𝙮 𝙎𝙚𝙡𝙚𝙘𝙩𝙞𝙤𝙣: 𝘽𝙚𝙮𝙤𝙣𝙙 𝘿𝙉𝘼 𝙈𝙪𝙩𝙖𝙩𝙞𝙤𝙣𝙨”
👉 Register here: bit.ly/BP-New-Front...


📆 𝙉𝙤𝙫𝙚𝙢𝙗𝙚𝙧 5 | 1–3 𝙋𝙈 𝙀𝙏
🎯 “𝙉𝙚𝙬 𝙁𝙧𝙤𝙣𝙩𝙞𝙚𝙧𝙨 𝙞𝙣 𝙏𝙝𝙚𝙧𝙖𝙥𝙮 𝙎𝙚𝙡𝙚𝙘𝙩𝙞𝙤𝙣: 𝘽𝙚𝙮𝙤𝙣𝙙 𝘿𝙉𝘼 𝙈𝙪𝙩𝙖𝙩𝙞𝙤𝙣𝙨”
👉 Register here: bit.ly/BP-New-Front...
This session will examine how MRD improves prognosis and patient outcomes and more!
💻 Register today for free: pcf.elevate.gocadmium.com

This session will examine how MRD improves prognosis and patient outcomes and more!
💻 Register today for free: pcf.elevate.gocadmium.com
biomodal develops technologies and analytics that serve as research tools for life scientists and clinical developers.
👉 Want a deep dive into what they do? Learn more: biomodal.com

biomodal develops technologies and analytics that serve as research tools for life scientists and clinical developers.
👉 Want a deep dive into what they do? Learn more: biomodal.com
👉 Register to this year’s Seminar: bit.ly/BP-New-Front...
👉 Check out last year’s seminar: www.bloodpac.org/beyond-ctdna
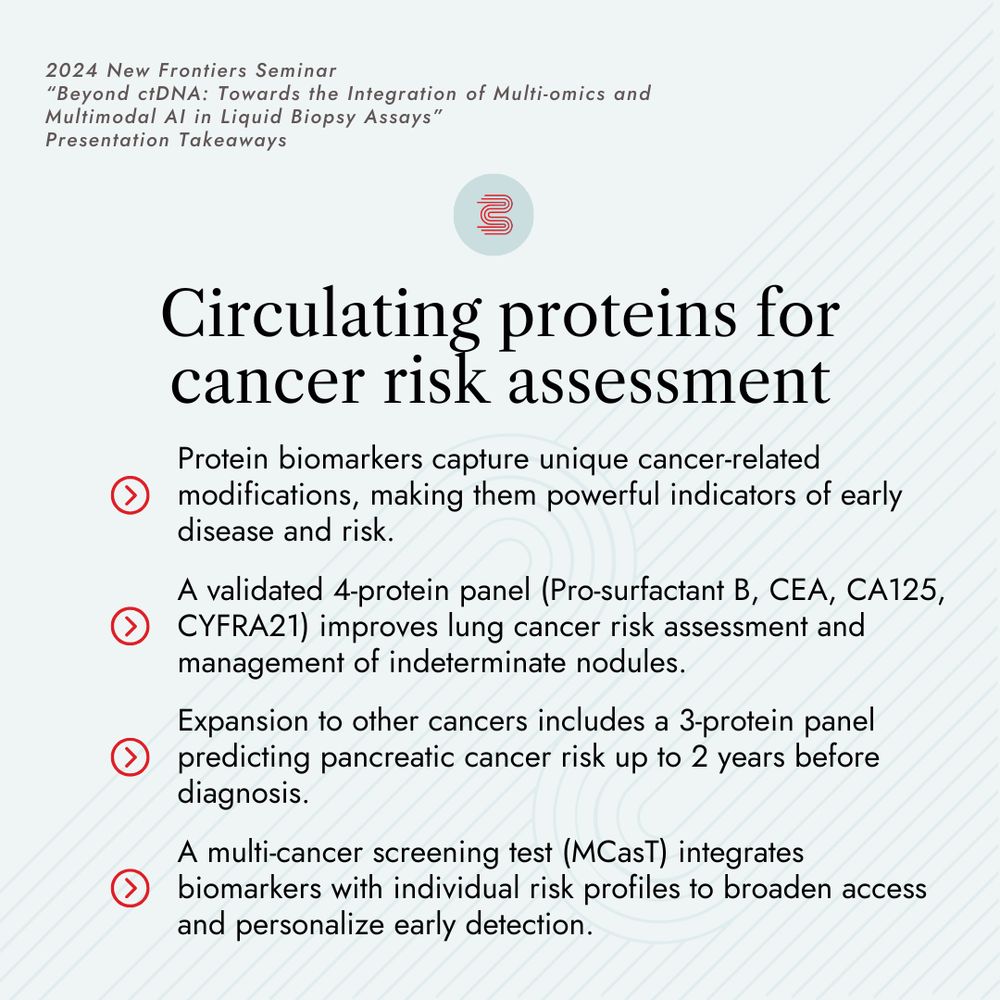
👉 Register to this year’s Seminar: bit.ly/BP-New-Front...
👉 Check out last year’s seminar: www.bloodpac.org/beyond-ctdna
👏 Thanks to the co-chairs & members.
Next up: a Berlin dinner at ESMO 2025 to advance global efforts. Read here: lnkd.in/giWczgsF

👏 Thanks to the co-chairs & members.
Next up: a Berlin dinner at ESMO 2025 to advance global efforts. Read here: lnkd.in/giWczgsF
Session: MRD in cancer - predicting relapse, guiding personalized care, and advancing research & clinical use.
✅ Free registration: pcf.elevate.gocadmium.com

Session: MRD in cancer - predicting relapse, guiding personalized care, and advancing research & clinical use.
✅ Free registration: pcf.elevate.gocadmium.com
At our Q3 meeting, the group aligned on study parameters and is planning an in-person kickoff with collaborators.
Excited for what’s coming up!


At our Q3 meeting, the group aligned on study parameters and is planning an in-person kickoff with collaborators.
Excited for what’s coming up!

We revisited goals, assessed priorities in analytical and clinical validation, and set the direction as we work toward our 2027 Summit.
On to what's next!


We revisited goals, assessed priorities in analytical and clinical validation, and set the direction as we work toward our 2027 Summit.
On to what's next!
At our Q3 meeting, they took time to realign and set the course for the next phase of collaborative efforts in advancing MRD clinical validation. More updates soon!


At our Q3 meeting, they took time to realign and set the course for the next phase of collaborative efforts in advancing MRD clinical validation. More updates soon!

