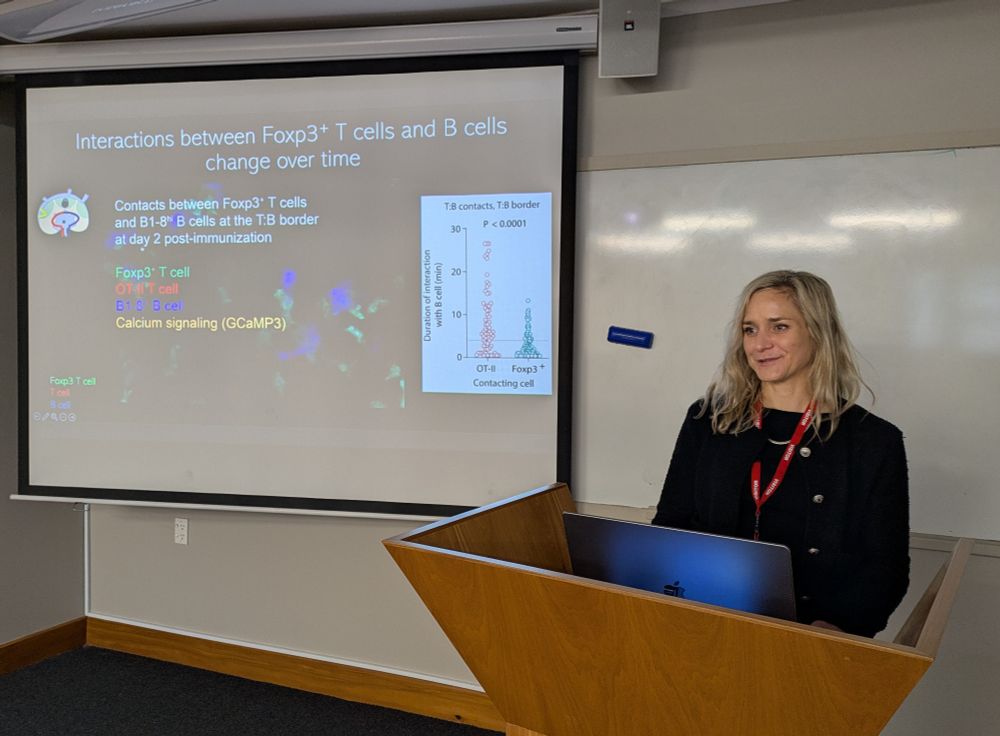
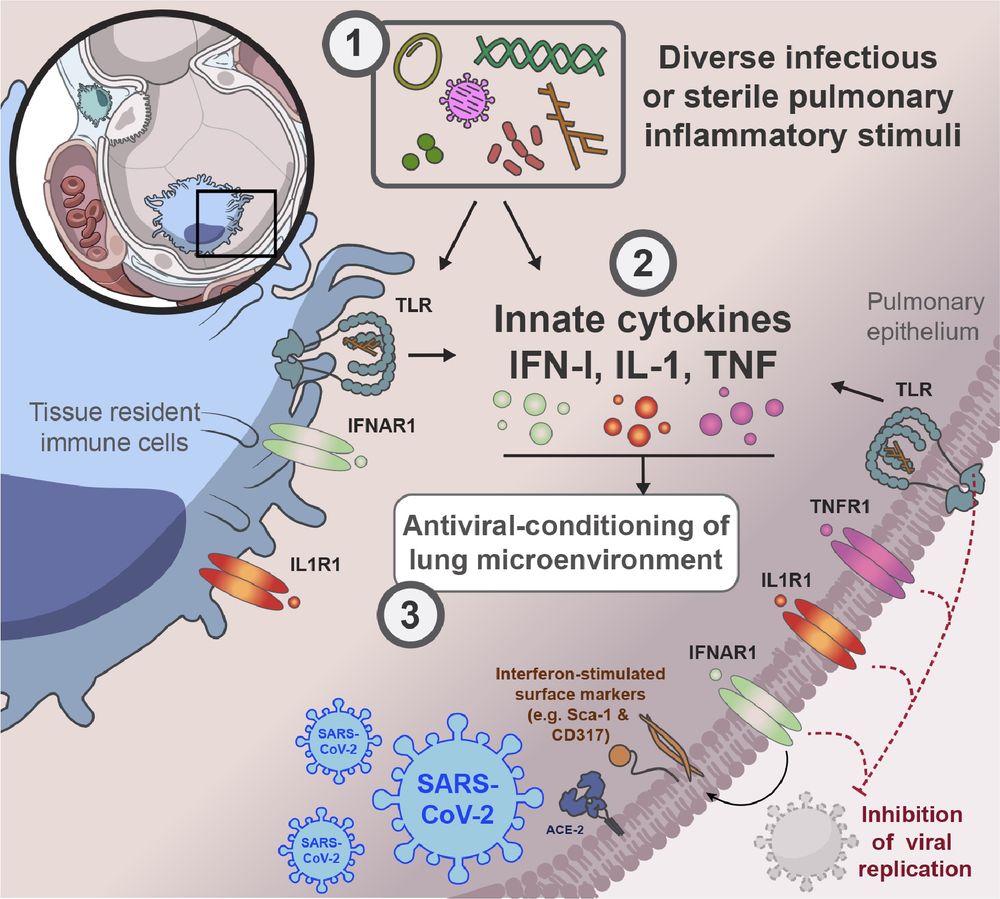
Malaghan Institute of Medical Research
@malaghan.bsky.social
82 followers
85 following
5 posts
New Zealand's world-class independent biomedical research institute, focusing on breakthrough discoveries in immunology and immunotherapy. Charity Reg. CC 10357
Posts
Media
Videos
Starter Packs
Reposted by Malaghan Institute of Medical Research
Reposted by Malaghan Institute of Medical Research
Reposted by Malaghan Institute of Medical Research
Reposted by Malaghan Institute of Medical Research